While prescription weight loss medications regularly make headlines for their effectiveness in shedding pounds, a new study suggests semaglutide can also lower heart failure risk.
Semaglutide is a glucagon-like peptide-1 (GLP-1) receptor agonist, an anti-obesity medication, that has shown promise in treating patients with heart failure. The STEP-HFpEF trial recently evaluated the effects of semaglutide on heart failure outcomes.
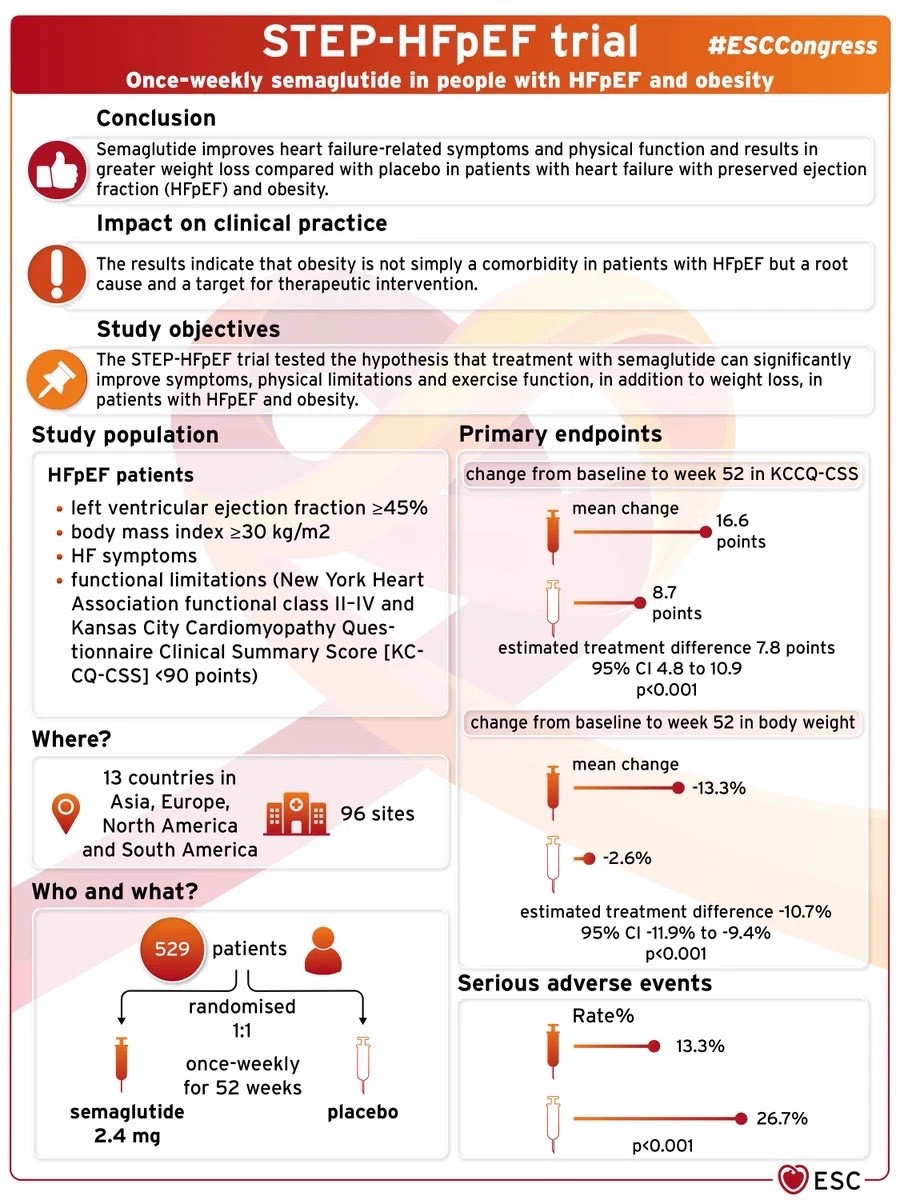
The STEP-HFpEF trial aimed to investigate the effects of semaglutide on people with heart failure. Specifically, it looked at those who had heart failure with a preserved ejection fraction (HFpEF), meaning their heart’s ability to relax was not as effective as in normal hearts. Researchers wanted to see if semaglutide could positively impact participants’ health.
The trial involved a large number of people with heart failure. Half of the participants were given semaglutide, while others received a placebo.
The population enrolled in the study had to meet the following criteria:
- Left ventricular ejection fraction (LVEF) > or equal to 45%
- Body mass index (BMI) > or equal to 30kg/m2
- Heart Failure symptoms
- Functional limitations NYHA functional class II-IV and KCCQ-CSS <90 points
The study demonstrated that semaglutide is superior to a placebo in improving body weight and quality of life at 52 weeks. The validity of this study is bolstered by its randomized, double-blind design, which minimizes bias. The large sample size (529 patients) also adds to its credibility, as it included participants across various demographics and regions, enhancing the generalizability of the findings.
STEP-HFpEF Trial’s Clinical Implications for Management of Heart Failure
New Treatment Option: The trial demonstrated that semaglutide can reduce the symptoms in patients with heart failure and preserved ejection fraction. This introduces semaglutide as a potential new tool in the treatment toolkit for healthcare professionals.
Improved Quality of Life: Semaglutide was shown to improve the overall quality of life for participants. This means that patients not only had better physical health outcomes, but they felt better and experienced a better sense of well-being.
Combination Therapy: The trial evaluated semaglutide in addition to standard heart failure treatments. This suggests that semaglutide could complement existing therapies, possibly enhancing their effectiveness when used in combination.
Targeting Heart Failure Subtype: The study focused on patients with heart failure and preserved ejection fraction. This subgroup of heart failure patients has limited treatment options, so semaglutide’s effectiveness in this group opens new avenues for treatment.
Preventive Aspect: By reducing the risk of hospitalizations and cardiovascular death, semaglutide might not only help manage existing heart failure but also have a preventive effect, potentially slowing down the progression of the disease.
Tailored Approaches: As treatment plans are personalized, the inclusion of semaglutide could allow doctors to tailor treatments more precisely to individual patients, potentially improving outcomes based on each patient’s needs.
Shared Decision-Making: Patients and their healthcare providers now have another conversation point when discussing treatment options. The positive outcomes from the trial empower patients to be more involved in deciding their course of treatment.
Research Direction: The success of semaglutide in heart failure opens avenues for further research into GLP-1 receptor agonists and their effects on other aspects of cardiovascular health, potentially leading to more innovative treatments.
Guideline Updates: The findings could influence the development of updated medical guidelines for heart failure management, incorporating semaglutide as a recommended treatment option.
Healthcare Policies: Positive trial results could prompt discussions among policymakers and health authorities about the inclusion of semaglutide in insurance coverage or national healthcare programs.
It’s important to note that while the STEP-HF trial’s findings are promising, ongoing research and real-world experience will provide a more comprehensive understanding of semaglutide’s role in heart failure treatment. Healthcare professionals will need to balance the benefits and potential risks when considering semaglutide for individual patients.
Limitations of the STEP-HFpEF Trial
Duration of Follow-Up: The trial followed participants for a specific period, and while it showed positive outcomes, the long-term effects of semaglutide still need to be studied over a more extended timeframe to ensure its safety and sustained effectiveness.
Sample Characteristics: The trial participants were carefully selected, which mightnot fully represent all people with HFpEF in real life. The study included certain demographics and health conditions, so the results might not apply equally to everyone.
Placebo Effect: Sometimes, people who know they’re taking a new medicine can feel better just because they believe the medicine is working. This is called the placebo effect and can influence study outcomes.
Generalization: The trial took place in specific locations with specific conditions. Results could vary in other regions or populations with different healthcare settings or lifestyles.
Combination with Other Treatments: The trial looked at the effects of semaglutide on top of usual care. It’s possible that the improvements observed were due to a combination of both, rather than semaglutide alone.
Lack of Long-Term Safety Data: While the trial assessed short-term safety, potential rare or long-term side effects might not have been fully uncovered yet.
Cost and Accessibility: The trial doesn’t delve into the cost or practicality of long-term semaglutide use, which could impact its real-world application.
Conclusion
While the STEP-HFpEF trial provides valuable insights, ongoing studies and real-world observations will help fill in the gaps and provide a more comprehensive understanding of semaglutide’s role in treating HFpEF.
The implications of this study for treatment are significant. Semaglutide could be a valuable addition to the therapeutic options for heart failure patients. Its ability to improve quality of life suggests it could become an integral part of the treatment strategy alongside conventional heart failure therapies.
However, as with any medical study, it’s important to consider potential limitations, such as the duration of follow-up and the need for further research to assess long-term safety and effectiveness. Overall, the use of semaglutide in heart failure patients represents a promising advancement in cardiac care.
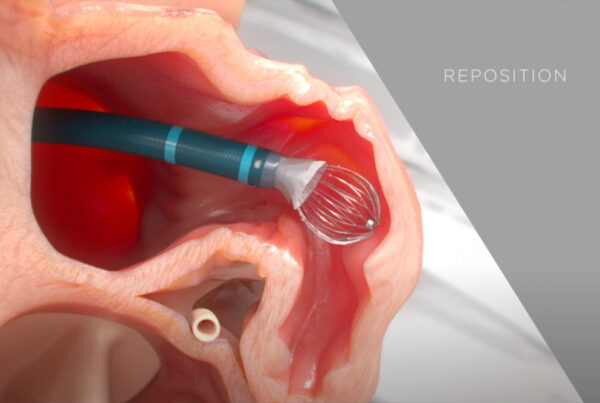
In our fast-paced and ever-evolving cardiovascular care landscape, breakthroughs like the Watchman Implant are changing the game, offering new hope to those grappling with NVAFib—a heart condition notorious for increasing the risk of stroke.
In this video, Dr. Imran Baig delves into the intricacies of NVAFib and the innovative solution provided by the Watchman Implant. He sheds light on how the Watchman Implant is transforming the lives of those living with NVAFib, including many of our Connected Cardiovascular Care patients.